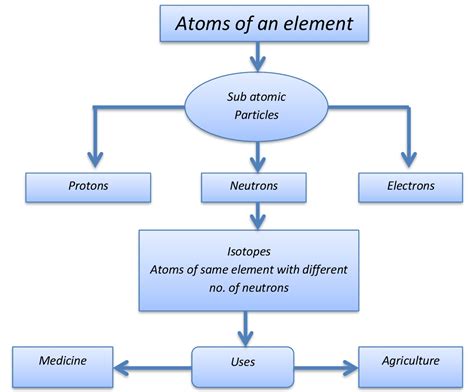
atoms are similar to subatomic particles ,sub atomic particles grade 8,atoms are similar to subatomic particles,This section explores atoms and isotopes covering, the structure of atoms, the development of the atomic model and isotopes. Structure of Atoms. Atoms are the basic building blocks of matter. .
The Most Sought-After AP Replica Models. Audemars Piguet has a rich history of iconic watch models. Many of these have been replicated with great precision and detail. One of the most popular AP replicas is the Royal .
Introduction
The world of atomic and subatomic particles is a realm that has fascinated scientists and researchers for centuries. From the discovery of the electron in 1897 to the modern-day exploration of quarks, leptons, and other subatomic particles, our understanding of the building blocks of matter has evolved considerably. While atoms form the foundation of all matter, they are themselves made up of even smaller particles, which are often called "subatomic particles." These particles play a crucial role in shaping the properties of elements, compounds, and everything in our universe.
In this article, we will delve into the nature of atoms and their subatomic constituents, discussing the similarities and differences between the two, the various types of subatomic particles, and how our understanding of them has expanded over the years.
Atoms: The Building Blocks of Matter
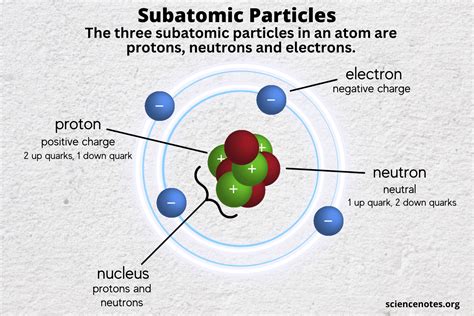
Before we dive into subatomic particles, let's first review what atoms are. Atoms are the fundamental units of matter, comprising three primary subatomic particles: protons, neutrons, and electrons. These particles combine in different ways to form different elements, which make up all substances in the universe.
An atom consists of a central nucleus that contains positively charged protons and uncharged neutrons, surrounded by negatively charged electrons that orbit the nucleus. The arrangement and number of these particles determine the chemical properties of the atom. For example, the number of protons in the nucleus defines the element (e.g., hydrogen has one proton, carbon has six protons), and the number of electrons governs the atom's ability to bond with other atoms.
The Subatomic Level: What Does It Mean?
The term "subatomic" refers to anything that exists beneath the atomic level, which is to say, particles that are smaller than atoms themselves. Atoms are composed of subatomic particles, and these particles—protons, neutrons, and electrons—are often studied to understand the behavior of matter on a very small scale. The subatomic world is a realm that exists beyond the ability of the naked eye to see and even beyond the resolution of ordinary microscopes. However, scientists have developed ways to explore this level of matter using particle accelerators and other advanced technologies.
At the subatomic level, particles do not behave in the same way as everyday objects. They often exist in quantum states, meaning their properties—such as position, velocity, and energy—can only be described in terms of probabilities. This is very different from the behavior of macroscopic objects we encounter in daily life, where properties are more easily defined.
Subatomic Particles: An Overview
At the core of every atom are the following subatomic particles:
1. Protons: These positively charged particles are found in the nucleus of an atom. They determine the atomic number of an element and give it its chemical properties. For example, hydrogen has one proton, and helium has two protons. Protons have a mass of approximately 1.6726 × 10^-27 kg.
2. Neutrons: Neutrons have no charge and are also located in the nucleus alongside protons. Neutrons help to stabilize the nucleus by offsetting the repulsive forces between positively charged protons. The mass of a neutron is slightly greater than that of a proton, approximately 1.6750 × 10^-27 kg.
3. Electrons: Electrons are negatively charged particles that orbit the nucleus of an atom. They are much lighter than protons and neutrons, with a mass of approximately 9.109 × 10^-31 kg. Electrons are involved in chemical bonding and play a key role in electricity and magnetism.
These three particles are the most well-known subatomic particles, but they are not the only ones that exist.
Smaller Than a Proton: The Quest for the Fundamental Particles
The discovery of protons, neutrons, and electrons marked the beginning of a deeper investigation into the nature of matter. However, as science advanced, physicists began to question whether there was something even smaller than protons and neutrons. The answer came with the discovery of quarks.
atoms are similar to subatomic particles $78K+
atoms are similar to subatomic particles - sub atomic particles grade 8